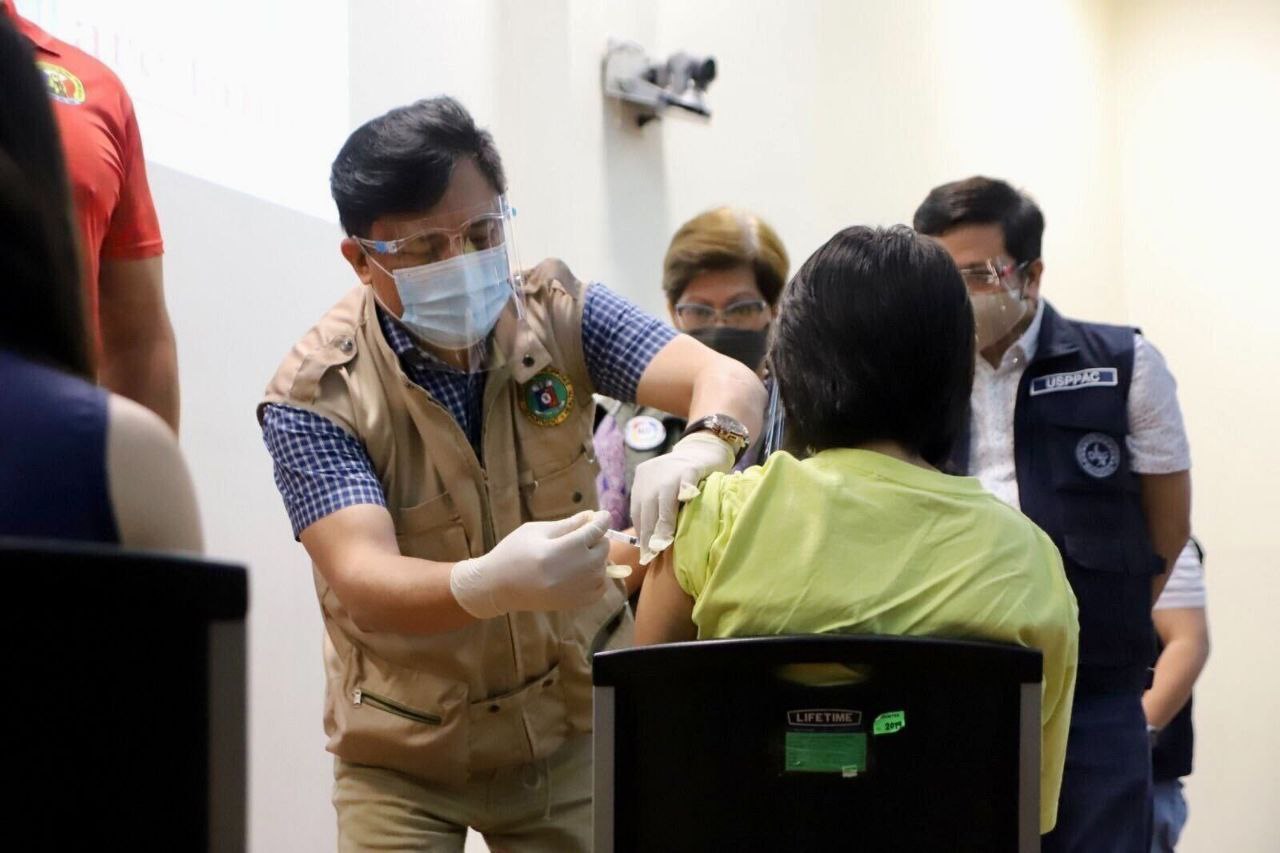
The Food and Drug Administration (FDA) has authorized the rollout of a second COVID-19 booster shot for senior citizens, immunocompromised individuals, and frontline health workers, the Department of Health said on April 13.
The DOH said the second booster dose should be administered at least four months after the first booster but it can be given earlier to moderate and severe immunocompromised patients subject to their doctor’s assessment.
Photo via Shutterstock
With this development, the DOH said the FDA recognized the waning efficacy of the vaccine in the specific populations after a few months.
The DOH added it is already drafting guidelines for the administration of second booster dose for the high-risk and vulnerable sector.
Earlier, the DOH said that it also requested the FDA to amend the emergency use authorization of COVID-19 vaccines to include booster jabs for children ages 12 to 17.
As of writing, only 12,477,480 individuals in the country have received their first booster dose of the coronavirus vaccine.
Thumbnail photo courtesy of the Department of Health